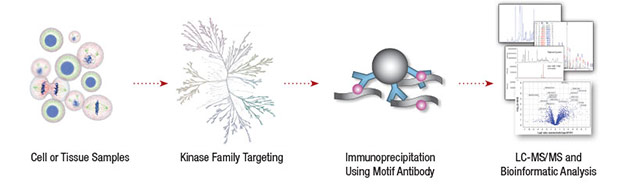
CST performed a global survey of tyrosine kinase activity in non-small cell lung cancer (NSCLC) to identify novel disease drivers (1). A phosphotyrosine antibody was used to enrich phosphorylated peptides from 41 NSCLC cell lines and 150 NSCLC tumors prior to LC-MS/MS analysis. The analysis identified 4551 phospho-tyrosine residues on more than 2700 proteins. The tyrosine kinase ALK (anaplastic lymphoma kinase) was among the top 10 candidates for follow up analysis.
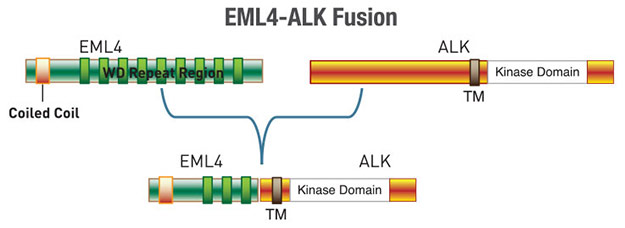
Further investigation revealed fusion of the N-terminus of EML4 (echinoderm microtubule-associated protein-like 4) with the C-terminus of ALK in some NSCLC cell lines and tumors. 3-7% of NSCLC patients express the fusion protein in their tumors indicating that the it is highly oncogenic (1-4). Cancer cells expressing the EML4-ALK fusion protein are sensitive to the small molecule ALK inhibitor crizotinib, and in 2011 the FDA approved crizotinib for the treatment of ALK positive NSCLC (2).
CST developed a highly specific and sensitive antibody, ALK (D5F3®) XP® Rabbit mAb #3633, which detects full length ALK and the EML4-ALK fusion protein. The FDA approved an immunohistochemistry (IHC) companion diagnostic test, which uses the ALK D5F3 clone licensed from CST (3). This will help physicians determine which NSCLC patients may be effectively treated with crizotinib.
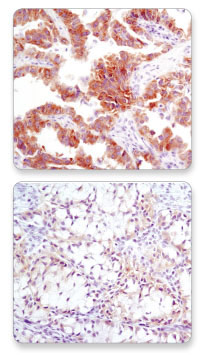
IHC analysis of paraffin-embedded human lung carcinoma with high (upper) and low (lower) levels of ALK expression using ALK (D5F3®) XP® Rabbit mAb #3633.