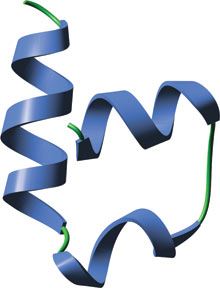
The VHLα domain of human VHL.
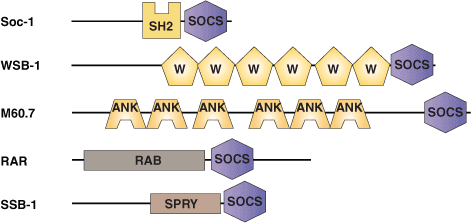
The SOCS box is an approximately 40 amino acid region of homology originally identified in members of the supressors of cytokine signaling (SOCS) family of proteins. The SOCS box is always located at the C terminus and appears to help target proteins for ubiquitination. The BC-box is a sub-domain common to both the SOCS box and the VHLα domain that facilitates binding to the Elongin BC complex. The Elongin BC complex also interacts with the von-Hippel Lindau (VHL) tumor suppressor protein to form the core of a larger VCB E3 ubiquitin protein ligase complex. In this context, the SOCS box/VHLα domain may be analogous to the structurally related F-box protein that links substrates to E3 complexes during ubiquitination. Five distinct types of N-terminal regions are associated with the C-terminal SOCS box. These include SH2 domains (SOCS proteins), WD40 repeats (WSB proteins), a SPRY domain (SSB proteins), Ankyrin repeats (ASB proteins), and a GTPase domain (RAR family).
The SOCS/VHLα domain consists of three α-helices that pack in a manner reminiscent of the so-called "folded-leaf" cluster, apart from the absence of one of the four α-helices. A helix from Elongin C fits into this gap to complete the four-helix cluster and create an arrangement where two pairs of helices pack perpendicular to one another.
| SOCS Domain Proteins | Binding Partners |
| Socs-1 | Elongin B/C |
| Socs-3 | Elongin B/C |