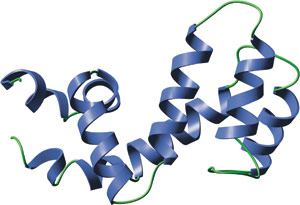
The RGS domain of RGS-4.
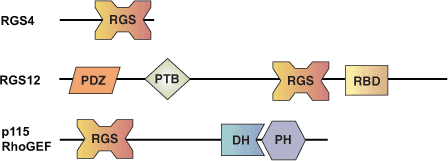
The RGS (Regulator of G protein Signaling) domain found in over 20 human proteins is approximately 120 amino acids in length. RGS domains act allosterically by stabilizing the transition intermediate of the GTP binding pocket of heterotrimeric G protein α subunits. This accelerates the intrinsic GTPase activity of the α subunit. The discovery of the RGS domain addressed a long recognized disconnect between the relatively slow intrinsic rate of hydrolysis of many heterotrimeric G proteins and the apparent cycling time for signaling processes that require G protein action. Heterotrimeric G proteins transmit signaling from seven transmembrane receptors that are activated by many important agonists including hormones, neurotransmitters, light and odorants. Proteins that encode RGS domains also modulate such signaling events by controlling the transmission time of each of these agonists.
The RGS domain is composed of nine α helices that form a right-handed anti-parallel four-helix bundle (α4-7) and a terminal bundle (α1-3, α8-9) containing the N- and C-termini of the domain. The interaction surface between RGS4 and Gai is formed by the loop between α3-4, the loop between α5-6, the end of α7 and the start of α8. All RGS domains share a highly similar tertiary structure, even members that share low amino acid identity. Interestingly, the structure of the RGS domain is quite different than that of the GAP domains for the monomeric G proteins such as p120 RhoGAP.
| RGS Domain proteins | Binding Partners |
| RGS-4 | GΑi, GΑq |
| p115 RhoGEF | GΑ12, GΑ13 |
| RGS-2 | GΑq |
| GAIP | GΑi, GΑq |